
Meet the Regulatory Innovation Platform Built for Medtech
Don't just centralize registrations, automate Submissions (including FDA eSTAR), gain AI-driven Regulatory Intelligence, Regulatory Strategy, Search and Report — all in one platform.
Don't just centralize registrations, automate Submissions (including FDA eSTAR), gain AI-driven Regulatory Intelligence, Regulatory Strategy, Search and Report — all in one platform.
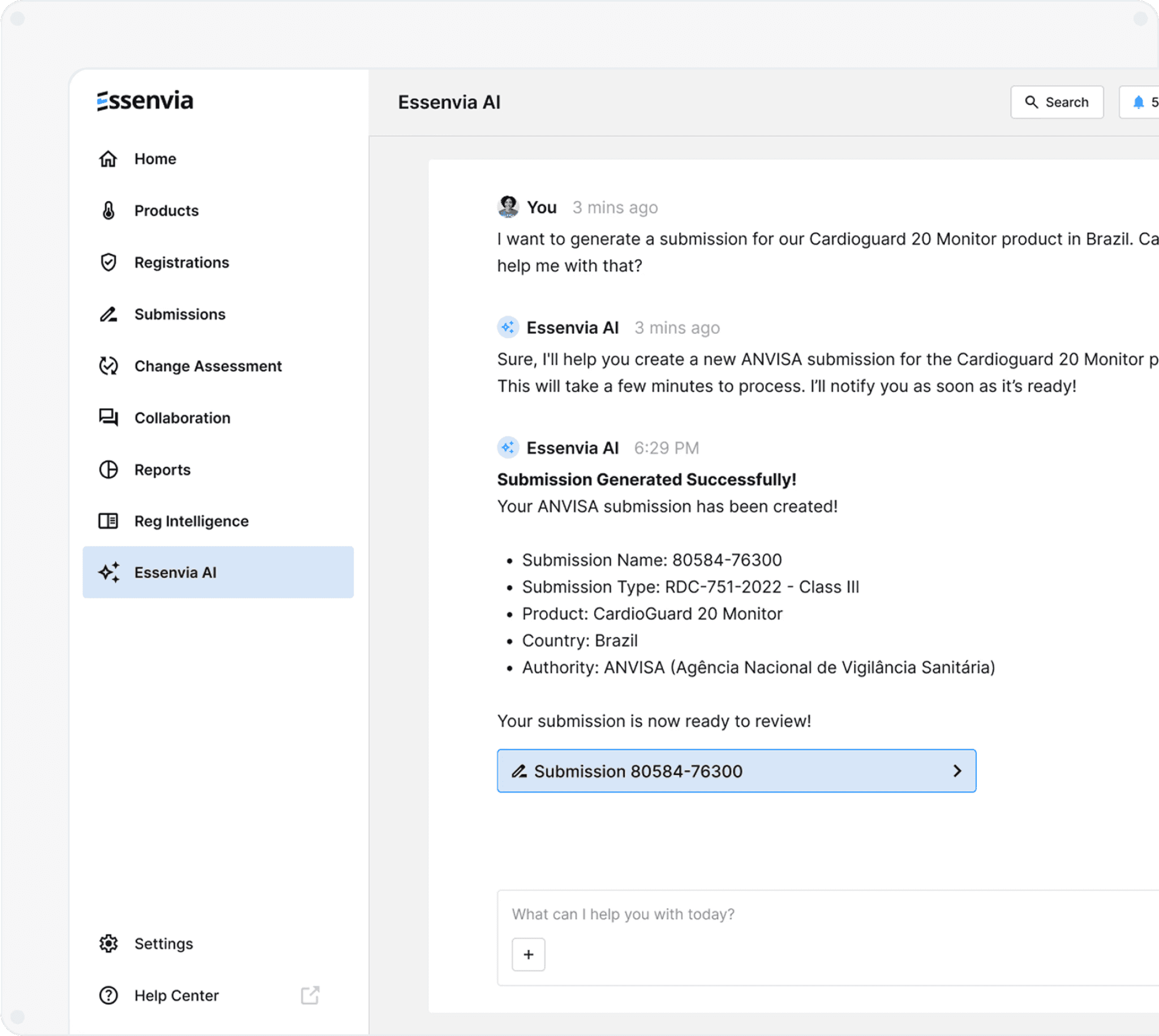

Meet the Regulatory Innovation Platform Built for Medtech
Don't just centralize registrations, automate Submissions (including FDA eSTAR), gain AI-driven Regulatory Intelligence, Regulatory Strategy, Search and Report — all in one platform.

We work with medtech regulatory teams worldwide
We work with medtech regulatory teams worldwide
Accenture
Trusted strategic implementation and advisory partner.

SOC 2
SOC 2 compliant for security and data protection.

GxP
Designed for GxP-regulated environments.

GxP
Designed for GxP-regulated environments.

21 CFR Part 11
Supports 21 CFR Part 11 requirements for records & signatures.

21 CFR Part 11
Supports 21 CFR Part 11 requirements for records & signatures.

GDPR
GDPR-aligned data protection across regions & systems.

GDPR
GDPR-aligned data protection across regions & systems.
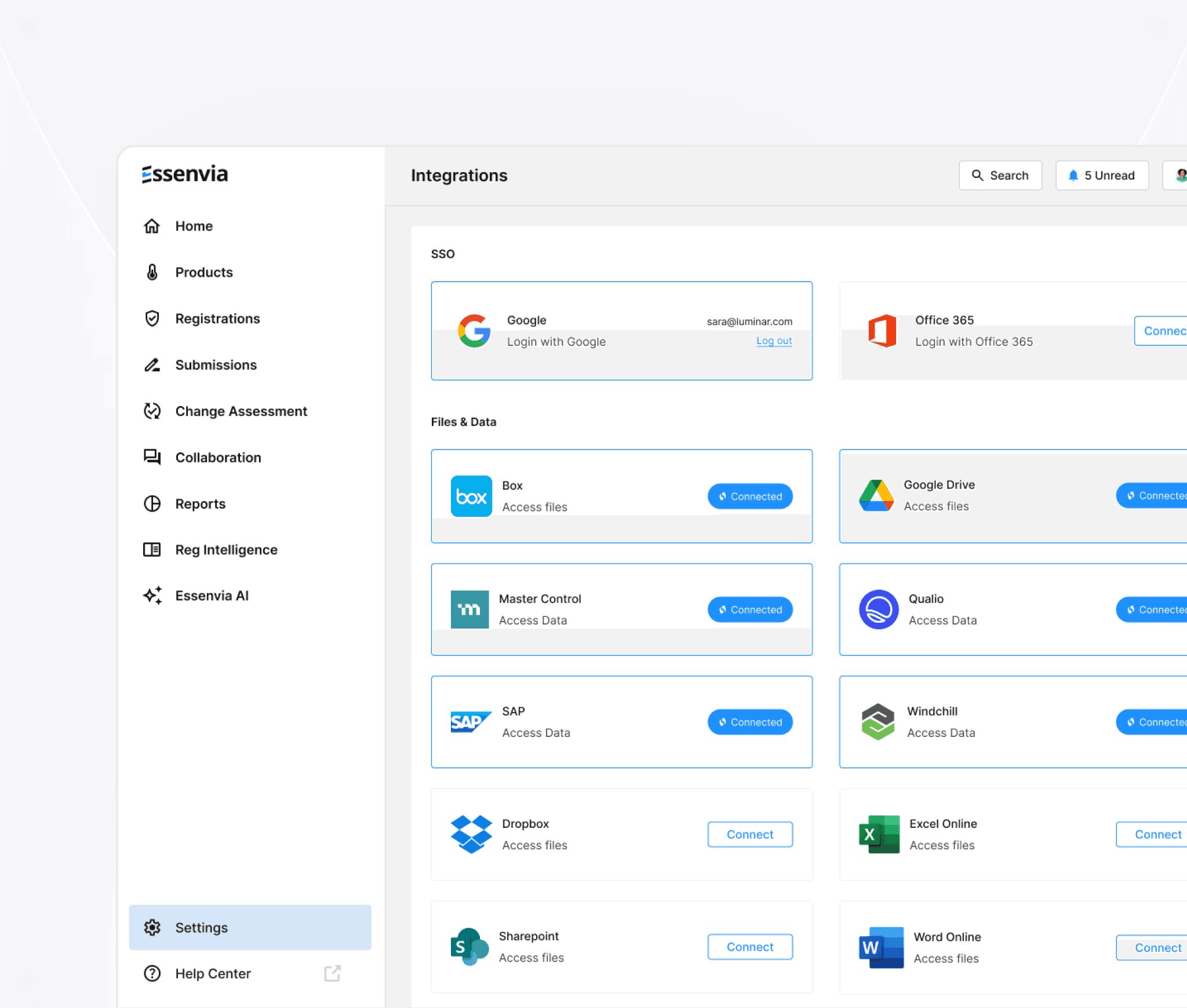
Packed with enterprise-grade features
Fast and Secure Setup
Draft in Hours
Stay Ahead of Changes
Track Everything

Fast and Secure Setup
Connect Essenvia to your existing systems (PLM, eQMS, ERP, file storage) quickly and securely with enterprise grade integrations.

Human-like ai chat generative ai feature
Deliver fast, natural conversations powered by AI without increasing your support team.


Smart system integration
Easily connect channels, tools, and workflows to power unified support across your ecosystem.


Fast and Secure Setup
Lorem ipsum dolor sit amet, consectetur adipiscing elit.

Fast and Secure Setup
Draft in Hours
Stay Ahead of Changes
Track Everything

Fast and Secure Setup
Connect Essenvia to your existing systems (PLM, eQMS, ERP, file storage) quickly and securely with enterprise grade integrations.


Human-like ai chat generative ai feature
Deliver fast, natural conversations powered by AI without increasing your support team.


Smart system integration
Easily connect channels, tools, and workflows to power unified support across your ecosystem.


Fast and Secure Setup
Lorem ipsum dolor sit amet, consectetur adipiscing elit.

Fast and Secure Setup
Draft in Hours
Stay Ahead of Changes
Track Everything

Fast and Secure Setup
Connect Essenvia to your existing systems (PLM, eQMS, ERP, file storage) quickly and securely with enterprise grade integrations.


Human-like ai chat generative ai feature
Deliver fast, natural conversations powered by AI without increasing your support team.


Smart system integration
Easily connect channels, tools, and workflows to power unified support across your ecosystem.


Fast and Secure Setup
Lorem ipsum dolor sit amet, consectetur adipiscing elit.

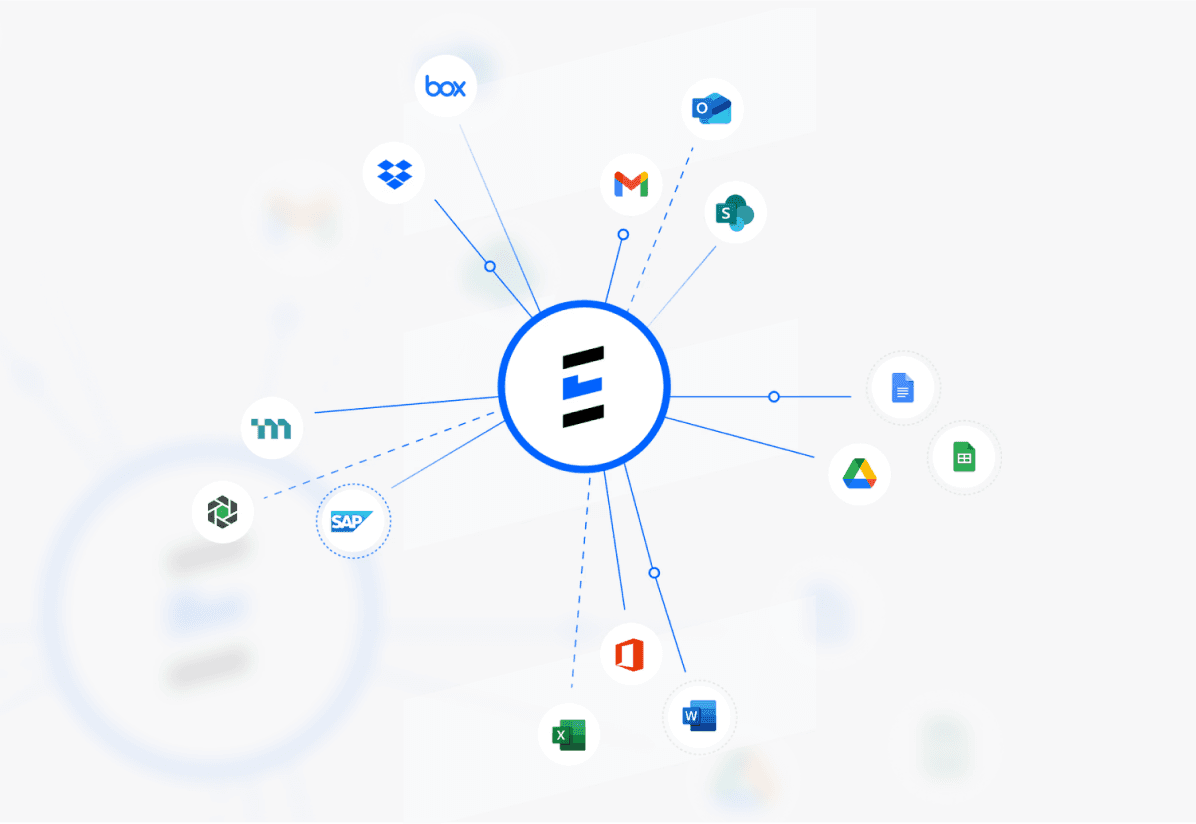
Essenvia RIMS AI Core Features

Connect, Migrate,
& Enrich Your Data
SSO integration with your existing systems. SOC2 compliant AI-powered migration and enrichment verified health authority datasources.

Connect, Migrate,
& Enrich Your Data
SSO integration with your existing systems. SOC2 compliant AI-powered migration and enrichment verified health authority datasources.


Connect, Migrate,
& Enrich Your Data
SSO integration with your existing systems. SOC2 compliant AI-powered migration and enrichment verified health authority datasources.
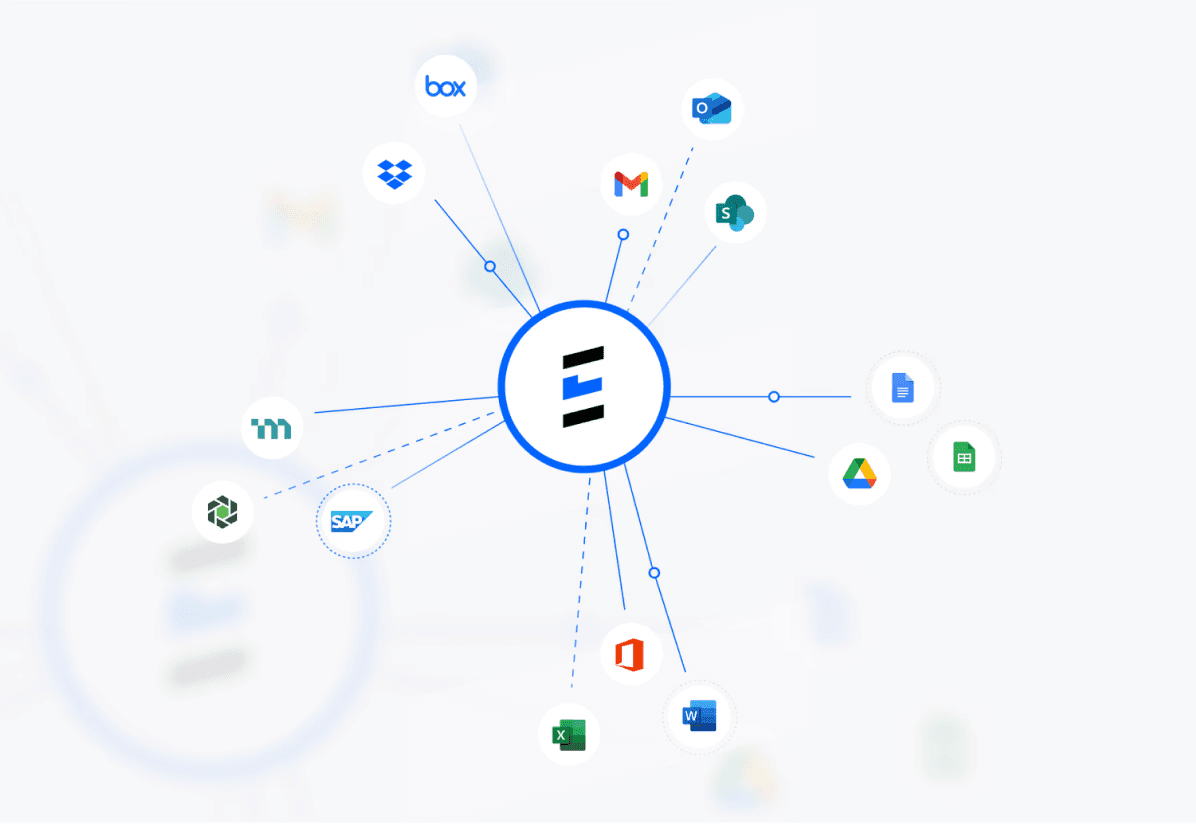


Submission AI and
Patented eSTAR Portal
Essenvia AI accelerates Global Submission Generation.


Submission AI and
Patented eSTAR Portal
Essenvia AI accelerates Global Submission Generation.


Submission AI and
Patented eSTAR Portal
Essenvia AI accelerates Global Submission Generation.

Regulatory
Intelligence
Monitor and keep up with rapidly evolving global regulations across your portfolio.
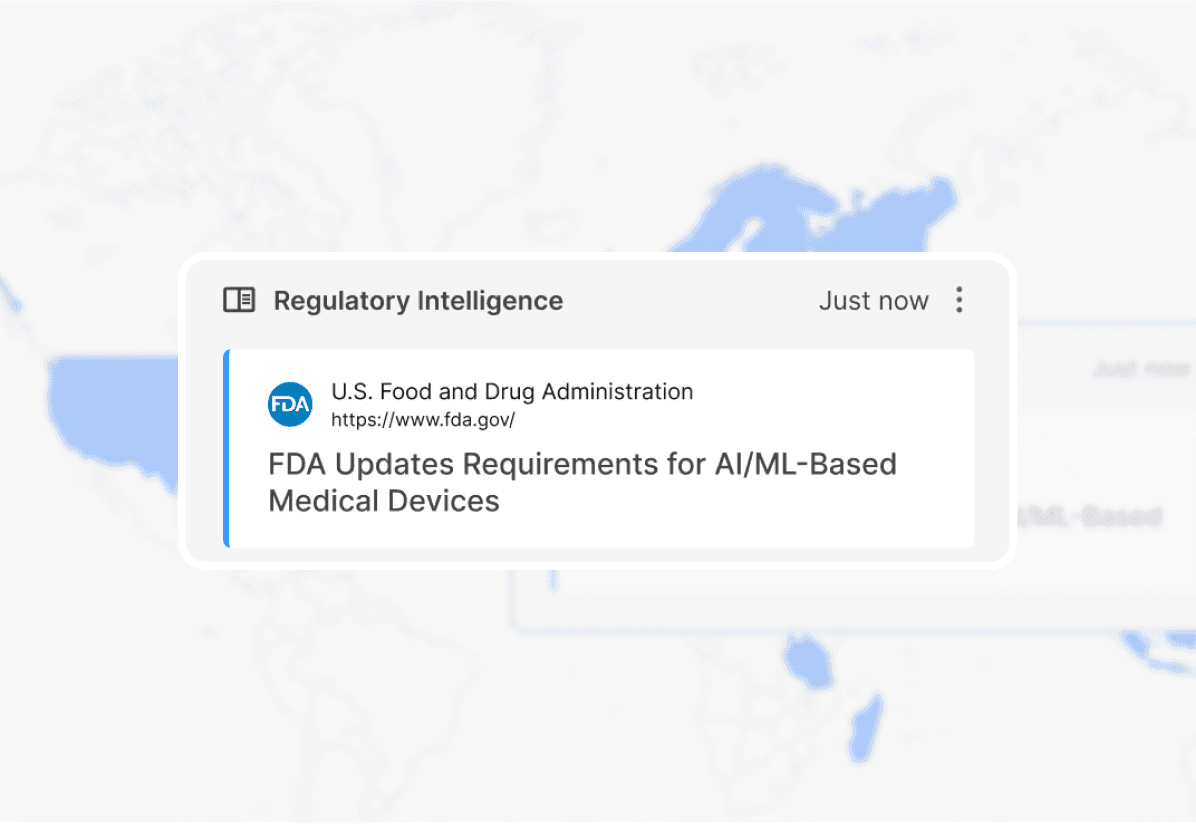

Regulatory
Intelligence
Monitor and keep up with rapidly evolving global regulations across your portfolio.


Regulatory
Intelligence
Monitor and keep up with rapidly evolving global regulations across your portfolio.

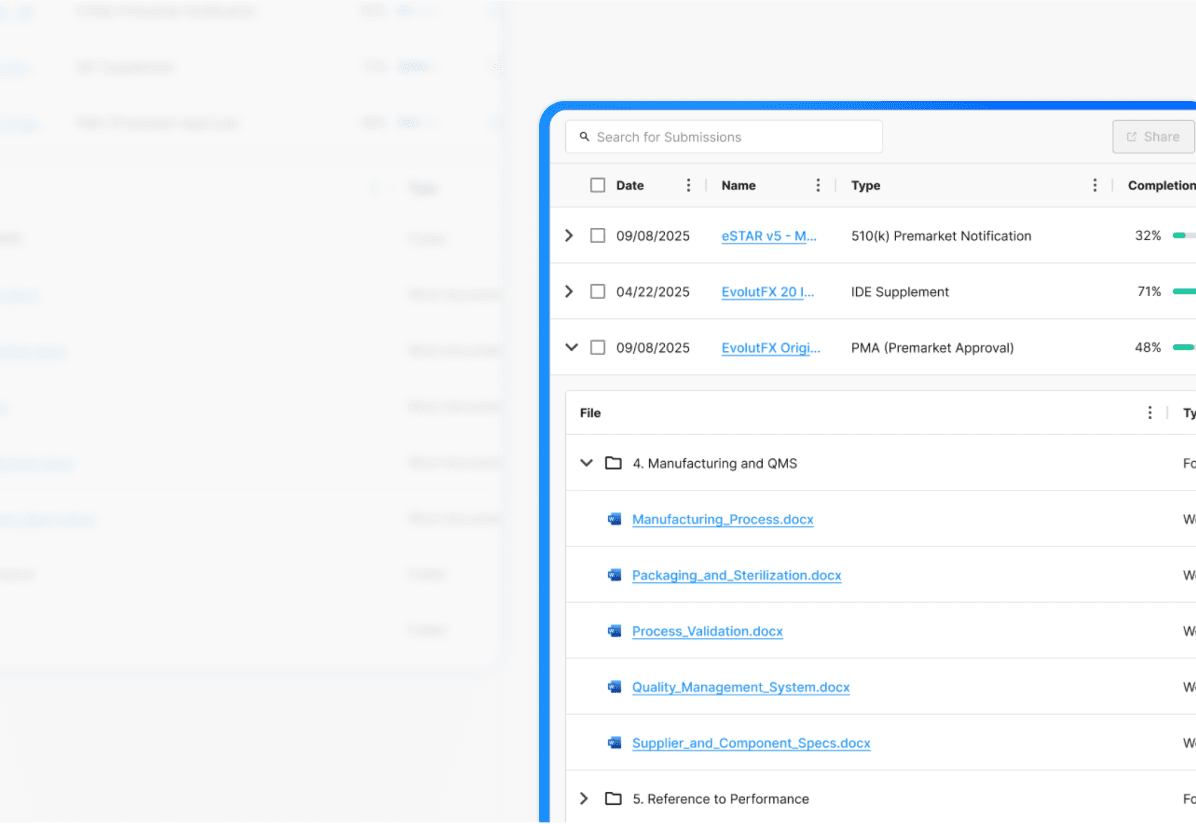

Your Single
Source of Truth
Complete storage and lifecycle tracking for submissions, registrations, and products with connections that power automation and impact analysis.


Your Single
Source of Truth
Complete storage and lifecycle tracking for submissions, registrations, and products with connections that power automation and impact analysis.


Your Single
Source of Truth
Complete storage and lifecycle tracking for submissions, registrations, and products with connections that power automation and impact analysis.
Trusted by Regulatory Affairs Teams Globally

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs
Essenvia’s collaborative eSTAR solution reduces a critical friction in submissions authoring and publishing. We're focused on optimizing big ticket submissions that involve multiple people.
Director Regulatory Affairs
Top 30 Medtech

The platform reduced our manual effort for submissions in Brazil and Mexico by 70%, freeing our time for strategic work. It is so easy and intuitive to use.
Sr. Regulatory Affairs Specialist
Top 3 Medtech

We're evaluating tools that have the potential to decrease manual work and increase productivity and margins - and this platform clearly demonstrated its potential to achieve that.
Sr. Director, Global Regulatory Submissions
Top 5 Medtech

I've seen a lot of so called AI softwares but very few are actually AI, and this [Essenvia] is the real deal.
Regulatory VP
Top 15 Medtech

The ability to have users manage specific eSTAR sections is really cool.
Manager Regulatory Affairs
Top 30 Medtech

Super user friendly, and I really like the modular approach.
Manager Regulatory Affairs
Top 40 Medtech


Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs
Essenvia’s collaborative eSTAR solution reduces a critical friction in submissions authoring and publishing. We're focused on optimizing big ticket submissions that involve multiple people.
Director Regulatory Affairs
Top 30 Medtech

The platform reduced our manual effort for submissions in Brazil and Mexico by 70%, freeing our time for strategic work. It is so easy and intuitive to use.
Sr. Regulatory Affairs Specialist
Top 3 Medtech

We're evaluating tools that have the potential to decrease manual work and increase productivity and margins - and this platform clearly demonstrated its potential to achieve that.
Sr. Director, Global Regulatory Submissions
Top 5 Medtech

I've seen a lot of so called AI softwares but very few are actually AI, and this [Essenvia] is the real deal.
Regulatory VP
Top 15 Medtech

The ability to have users manage specific eSTAR sections is really cool.
Manager Regulatory Affairs
Top 30 Medtech

Super user friendly, and I really like the modular approach.
Manager Regulatory Affairs
Top 40 Medtech


Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs
Essenvia’s collaborative eSTAR solution reduces a critical friction in submissions authoring and publishing. We're focused on optimizing big ticket submissions that involve multiple people.
Director Regulatory Affairs
Top 30 Medtech

The platform reduced our manual effort for submissions in Brazil and Mexico by 70%, freeing our time for strategic work. It is so easy and intuitive to use.
Sr. Regulatory Affairs Specialist
Top 3 Medtech

We're evaluating tools that have the potential to decrease manual work and increase productivity and margins - and this platform clearly demonstrated its potential to achieve that.
Sr. Director, Global Regulatory Submissions
Top 5 Medtech

I've seen a lot of so called AI softwares but very few are actually AI, and this [Essenvia] is the real deal.
Regulatory VP
Top 15 Medtech

The ability to have users manage specific eSTAR sections is really cool.
Manager Regulatory Affairs
Top 30 Medtech

Super user friendly, and I really like the modular approach.
Manager Regulatory Affairs
Top 40 Medtech


Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs
Essenvia’s collaborative eSTAR solution reduces a critical friction in submissions authoring and publishing. We're focused on optimizing big ticket submissions that involve multiple people.
Director Regulatory Affairs
Top 30 Medtech

The platform reduced our manual effort for submissions in Brazil and Mexico by 70%, freeing our time for strategic work. It is so easy and intuitive to use.
Sr. Regulatory Affairs Specialist
Top 3 Medtech

We're evaluating tools that have the potential to decrease manual work and increase productivity and margins - and this platform clearly demonstrated its potential to achieve that.
Sr. Director, Global Regulatory Submissions
Top 5 Medtech

I've seen a lot of so called AI softwares but very few are actually AI, and this [Essenvia] is the real deal.
Regulatory VP
Top 15 Medtech

The ability to have users manage specific eSTAR sections is really cool.
Manager Regulatory Affairs
Top 30 Medtech

Super user friendly, and I really like the modular approach.
Manager Regulatory Affairs
Top 40 Medtech


Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Essenvia RIM is easy to use and fast to setup. We save at least 20% of our time with using Essenvia's capabilities. Our experience with other RIMS tools made day-to-day operations more complex did not fit into our workflows”
Sejal Chitre
Director Global Regulatory Affairs


Essenvia greatly shortens the time to submission. It saved us countless hours in managing files, edits an even major updates at the last minute for very complex submissions. We can’t live without using the platform now.”
John Landsdown
Director of Quality, Regulatory Affairs & Compliance


We completed and successfully submitted our first FDA eSTAR submission using Essenvia. Thanks to this Platform our team was able to work together in one single place at any time to author, edit, and review the submission."
Marta Stepien
VP of Clinical, Regulatory and Quality Affairs

Learn More About the Essenvia RIM Platform
Contact us for a Demo

Learn More About the Essenvia RIM Platform
Contact us for a Demo

Learn More About the Essenvia RIM Platform
Contact us for a Demo





